The Click in Joints
- by Joanna Blair
- •
- 02 Dec, 2018
- •
Why, What Happens and Physiological Theories

An articular release can occur with or without an accompanying noise and in general, the occurrence helps increase the distance between articular joint surfaces to free joint motion and release joint tension (12). It is worth noting that not all noise from a joint resembles an articular release (12).
Difference Between Joint Manipulation and Mobilisation
Joint and spinal manipulation and mobilisation are two techniques that are commonly used by osteopaths, chiropractors and physiotherapists to treat joint pain and dysfunction.Spinal manipulative therapy (SMT) via mobilisation and manipulation techniques are also commonly used for the treatment of lower back pain and dysfunction (15).
Mobilisation involves passive rhythmic and repetitive movements in a range of motion against a 'restrictive barrier' within stiff joints (9, 15). It is a gentle technique, using passive range of motion that is commonly used as an extension of testing of either a single joint or a group of spinal segments (9). The techniques should be performed at low velocities, where the force and amplitude used is controlled (15).
Theories For What Causes Joint Crepitus or an Audible Click
Cavitation is the term used to describe the formation of vapor and gas bubbles (also known as cavities) within (synovial) fluid through a local reduction in pressure. The reduced pressure occurs through a mixture of gas and liquid. A break in the surface of gas within the synovial fluid vaporizes, gas is released from the solution and the collapse of vapor cavities gives rise to the articular noise (12).
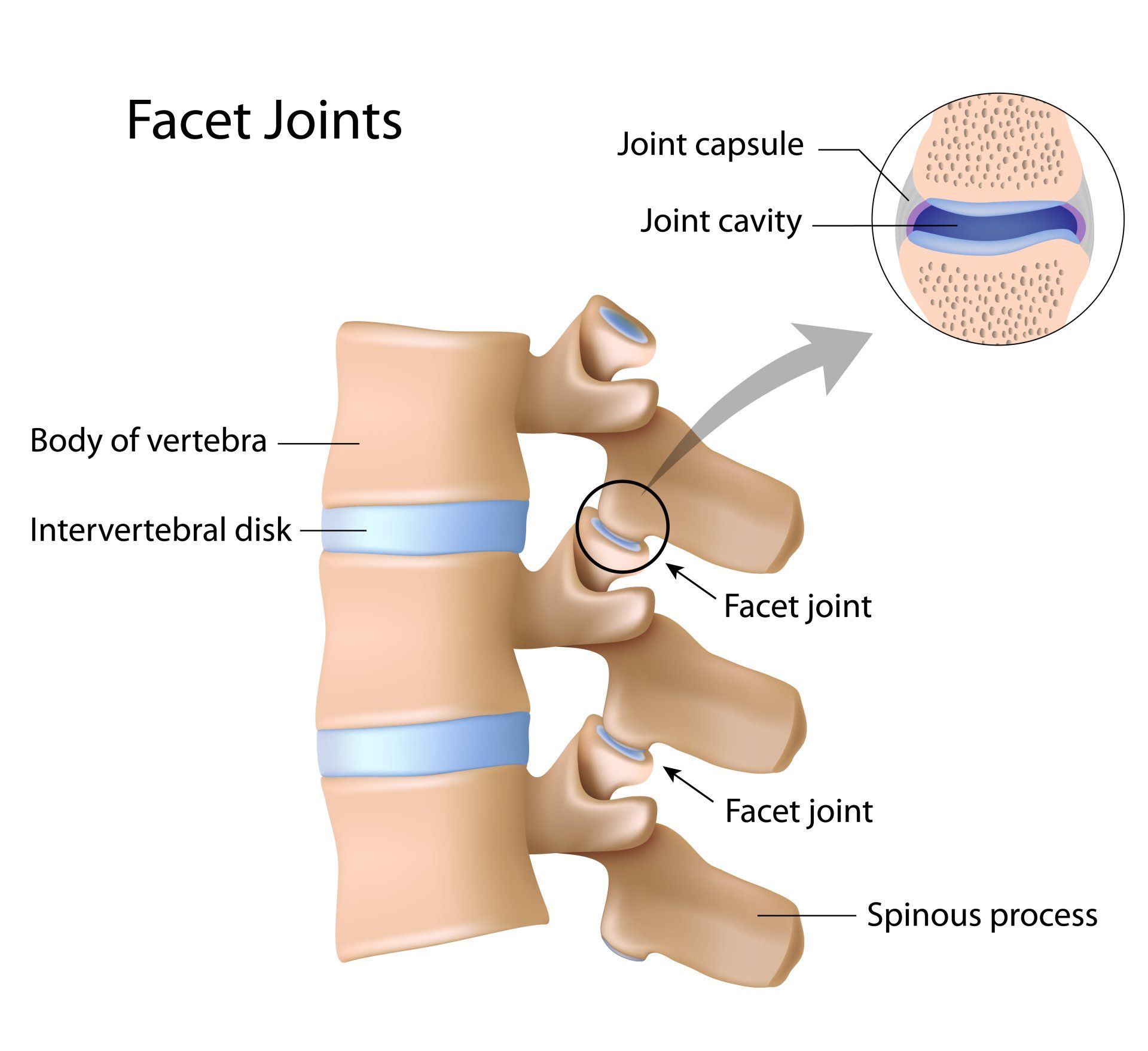
Unsworth et al (1971) found that joint cavitation occurence is dependent on joint space. The larger the joint space e.g. the hip, the less likely that the joint will pop, just as the smaller the joint space, the more likely it is that the articular release will be audible, e.g. the finger (12).
Theories for Physiological Effects of the HVLAT - What The Science Says
According to Fryer et al., (2009), it is suggested that manipulation and mobilisation can have a significant effect of increasing the threshold of pain when compared to a control group. This is controversial compared to other studies which otherwise failed to show a significant decrease in pain following spinal manipulation (15).
It has been suggested that peripheral joint manipulation increases the levels of serotonin (5-HT) which acts on the spinal 5-HT1A (serotonin 1A receptor) to produce antihyperalgesia (reduce pain)(13). Avila-Rojas et al., (2015) also suggest that 5-HT5A receptors reduce pain processing in the spinal cord as 5-HT and 5-CT (serotonin neurotransmitters) and reduce neuropathic pain through the activation of 5-HT5A and 5-HT1A/1B/1D receptors.
Previous evidence from studies supports the notion that hypoalgesia (a reduction in pain) has been observed following spinal manipulative therapy (SMT) by activating the descending pathways in the brain inhibitory systems, mediated via the midbrain dorsal periaqueductal grey region (dPAG) (figure 1) (15).
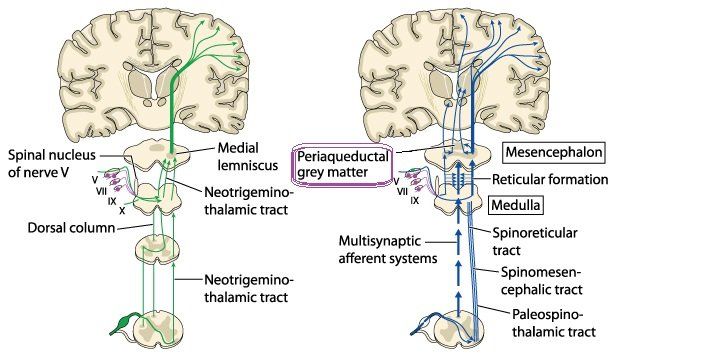
Descending pathways influence pain perception. The periaqueductalgray (PAG) matter (Figure 1) is part of the brain which when stimulated produces profound analgesia via the descending PAG pathways. Stimulation of the dorsal PAG (dPAG) in the brain selectively reduces pain to mechano-nociception (perceived pain during movement), whereas temperature nociception is modulated via the ventral PAG (vPAG) (12).
Scientists' fascination of the periaqueductal gray (PAG) and its relation to pain first started in 1969 when it was reported that electrical stimulation of the midbrain (PAG) produced 'profound' analgesia in the male rat (9).
The periaqueductal gray (PAG) is divided into four longitudinal columns; ventrolateral (vlPAG) lateral (lPAG), dorsolateral (dlPAG), dorsomedial (dmPAG) and is postulated to have an affect on behavioural control. It is the primary control centre for descending pain modulation and has enkephalin producing cells, a pentapeptide that regulates nociception in the body, and helps to suppresses pain (10).
Sterling et al. (2001) measured changes in pain and sympathetic outflow by comparing a C5/6 HVLAT to a sham manipulation (via manual contact but with no movement). The authors demonstrated HVLAT produced mechanical hypoalgesia measured by an increase in pain pressure threshold and increased sympathetic outflow, reduced blood flow, decreased skin temperature, and increased skin conductance.
However, there was no alteration to thermal pain thresholds. The selective mechanical anti-nociception and sympathoexcitation supports the theory that the mechanism of effect is due to the activation of the dPAG descending pain mechanism (11).
Vincenzino et al. (1998) conducted similar experiments on subjects with epicondylitis and showed again that cervical spine HVLAT lead to selective analgesia to mechanical stimulus and sympatho-excitation, adding further weight to the argument that spinal manipulation may influence the perception of pain by activation of the descending dPAG (12).
Skyba et al., (2009) suggests that joint manipulation activates the lateral periaqueductal gray (lPAG), glutamate excitation of the lateral PAG produces non-opioid analgesia, sympathetic excitation and motor facilitation. Stimulation of the PAG or RVM also increases the release of 5-HT (mentioned above) and nonadrenaline along the spine and electrical stimulation of the descending inhibitory pathways exerts inhibitory effects on dorsal horn neurons and produces antinociception (13).
HVT and Stretch Reflex
Clark et al., (2011) found that a single spinal manipulation does not alter corticospinal or stretch reflex excitability (muscle contraction in response to stretching ) of the erector spinae muscles in patients with chronic lower back pain when assessed 10-minutes following manipulation. They did find, however, that participants exhibiting an audible response exhibited a significant reduction (by 19.2%) in the stretch reflex compared to when spinal manipulation did not cause an audible joint sound with a 9.7% increase (4).
Spinal Manipulation Demonstration:
Substance P has been widely studied and is released in the dorsal horn of the spinal cord by C fibres which is needed for the central transmission of nociceptive input (11). It is thought that β-endorphins exert their anti-nociceptive influence by reducing the effectiveness of substance P in the dorsal horn and decrease afferent nociceptive input to higher centres (13).
Various studies have investigated whether a reduction in pain (hypoalgesia) following spinal manipulative therapy (SMT) involves the release of endorphins. Vernon et al., (1986) researched this theory by measuring plasma b-endorphin levels in 21 subjects following a cervical spine HVLAT. Only the HVLAT group found a small but statistical increase in plasma 𝛽-endorphin levels in the experimental group.
However, subsequent studies into theβ-endorphin system failed to show any significant effect. Two subsequent studies demonstrated findings contradicting those of Vernon et al. (1986) by Christian et al. (2) and Sanders et al. (12) and both failed to find increases in plasma β-endorphin concentrations in experimental groups with respect to sham and control groups following spinal manipulation (18).
References
1. Avila-Rojas, S. H., Velázquez-Lagunas, I., Salinas-Abarca, A. B., Barragán-Iglesias, P., Pineda-Farias, J. B., Granados-Soto V. (2015) Role of spinal 5-HT5A, and 5-HT1A/1B/1D, Receptors in Neuropathic Pain Induced by Spinal Nerve Ligation in rats, 5; 1622: 377-85.
9. Loyd, D. R., Morgan, M. M., Murphy, A. Z. (2008) Sexually Dimorphic Activation of the Periaqueductal Gray–Rostral Ventromedial Medullary Circuit During the Development of Tolerance to Morphine in the Rat, Eur J Neuroscience; 27(6): 1517.